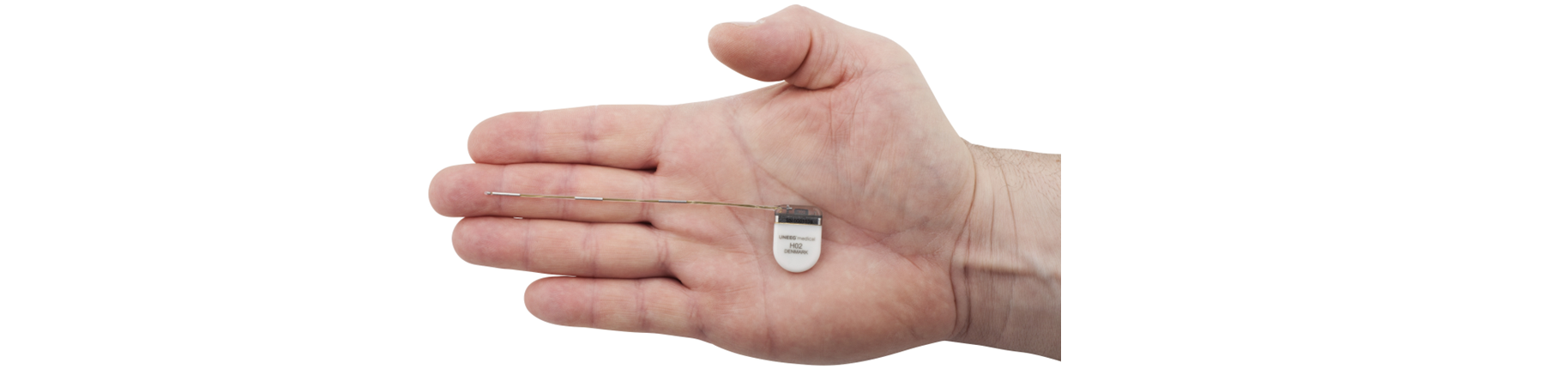
UNEEG medical Achieves EU Approval for 3-Year Continuous Use of UNEEG SubQ Implant – Further Strengthening its EpiSight Solution for Epilepsy Monitoring
COPENHAGEN, June 11, 2025 – UNEEG medical, a Danish company at the forefront of neuro- and epilepsy science, proudly announces the EU approval for 3-year continuous use of its subcutaneous implant, which is a significant milestone for the UNEEG EpiSight solution.
The minimally invasive UNEEG SubQ implant, a central component of our EEG monitoring solution, is designed to be placed under the skin. The SubQ implant measures patients’ EEG and communicates with an external recorder, which receives and stores the recorded data. The signal quality of the UNEEG SubQ is comparable to scalp EEG and has demonstrated low impedance and high stability over years. This reinforces the suitability of subcutaneous EEG technology for long-term seizure monitoring.
Traditional EEG recordings in Epilepsy Monitoring Units typically last less than 5 days, but the UNEEG SubQ implant allows for continuous EEG monitoring for up to 3 years. This extended monitoring period provides invaluable clinical insights based on real-life data capture, significantly enhancing patient treatment.
Approximately 30% of people with epilepsy continue to experience uncontrollable seizures despite trying multiple anti-seizure medications. These patients exhaust standard diagnostic pathways, remaining with unresolved diagnostic gaps. UNEEG’s EpiSight solution addresses these uncertainties by providing longitudinal, objective data recorded during patients’ everyday lives.
Martin Stenfeldt, CEO of UNEEG medical, remarks, "This approval represents a pivotal advancement in epilepsy monitoring, offering patients and healthcare providers a reliable, long-term solution for managing epilepsy." He adds, "Extending the end use of the implant to 3 years meets the demands of our patients and healthcare professionals for ultra long-term monitoring. As epilepsy is a dynamic and episodic disease, constant ultra long-term monitoring is essential to optimize treatment continuously."
