Recorder - collects and
stores EEG data
The 24/7 EEG SubQ recorder is the wearable, external part of our EEG monitoring solution. The recorder has been designed to be discrete and user-friendly.
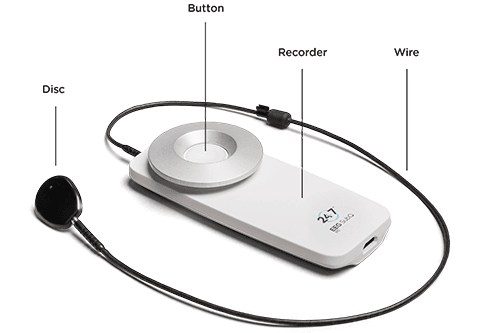
The 24/7 EEG SubQ recorder retrieves and stores EEG data and powers up the implant. The recorder is small and discrete, and records the patient’s EEG data subcutaneously day and night for up to 15 months.
The patients continue to live their regular life while wearing a discrete recorder without feeling constrained in daily activities. Hospitalisation is not needed during monitoring.
Powers implant via inductive link
EDF+ file format
Min 24 hour battery
30+ days storage capacity

The disc element of the recorder is attached on the skin behind the patient’s ear, placed directly above the implant. The recorder is attached to the clothes with a small magnet keeping the recorder discretely hidden under the clothes. A non-magnetic version is available.
The button serves several purposes. It switches the recorder on and off and also enables the patient to register an event. The event will be visible in the EEG data.
EEG data are automatically sent to the hospital once a day, allowing for on-demand access to patients’ objective data on electrographic seizures.
This on-demand data access gives the physician the flexibility to assess the treatment effect on seizure activity at any time, and has the potential to advance clinical decision-making.
High patient compliance
Our solution has been designed to be discrete, simple and user-friendly to encourage high patient compliance.

It includes a wearable recorder that can be placed under the shirt by a magnet and a tablet that automatically sends data to the hospital.
In a case study over 230 days, the patient recorded an average of over 20 hours per day. In addition, an average adherence rate of 18.1 hours per day was seen in a 3-month study.
Discrete
Simple
User-friendly
FAQ on recorder
Below you can find answers to the most frequently asked questions about the wearable recorder.

The implant measures the EEG when connected to the recorder. If the patient does not wear the recorder, EEG data will not be collected or stored. The recorder also serves to power up the implant.
It is recommended that the solution be used as much as possible, both day and night, to ensure that all seizures are measured.

The recorder is small and discrete. Patients can go on living their regular life without feeling constrained in daily activities.

This is up to the doctor and patient to decide. The implant is designed to remain implanted up to 15 months, but monitoring may not be needed for that long. When monitoring is no longer needed, the implant is removed under local anaesthesia.
Technical specifications
The 24/7 EEG SubQ recorder consists of three parts – a recorder, a disc and a wire.
Min 24 hours
30+ days
EDF+ is the file format of exported EEG files
Length: 89.9 mm
Width: 37.5 mm
Thickness (without Attachment Magnet): 10.9 mm
Thickness (with Attachment Magnet): 15.6 mm
Polycarbonate/Acrylonitrile Butadiene Styrene (PC ABS)
Material grade: Sabic CYCOLOY HC1204HF.
Weight (without Attachment Magnet and wire): 37.1g
Diameter: 15.9x20.4 mm
Thickness: 3 mm
Moulded in epoxy
Weight (with wire): 2.9g
Length: 360 mm
Outer diameter: 1.4 mm
Cable: Silicone
Bend reliefs: Polyamide (PA)
Data Analysis
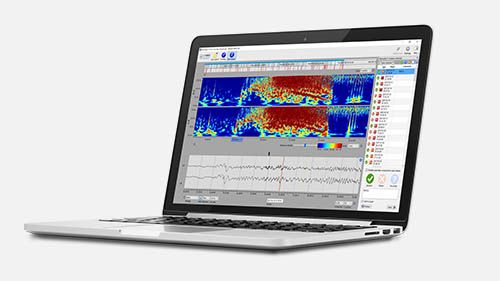
The recorded EEG data are automatically transferred by the provided tablet to UNEEG EpiSight, which is a highly advanced review system dedicated for 2 channel data.
The system highlights suspected seizures for quick expert review and allows the healthcare professional to make easy-to-read reports with the results.
Benefits of our solution
Reliable measurement of treatment effect
Rapid, automated analysis
On-demand EEG data access
Geographical barriers are removed
Proven high adherence rate
New insights with real-life data
Motivate patients
Ultra long-term EEG monitoring
High data quality over time

