Driving brain health therapy forward


2026
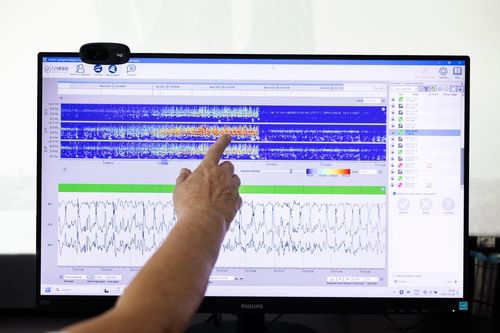
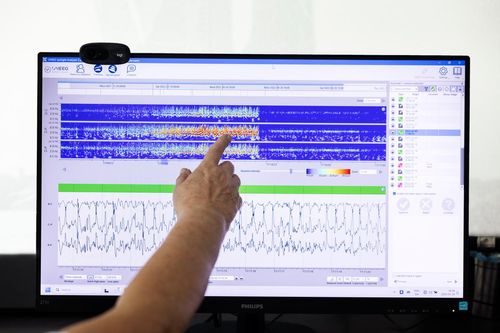
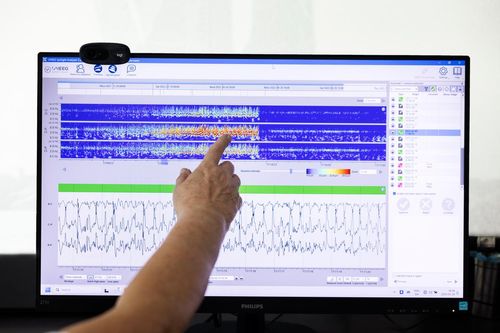
UNEEG has now accumulated more than 450,000 hours of real-world EEG, including 10,000 annotated seizures and 150,000 hours of sleep data. This unprecedented dataset continues to grow.
2025
An investor-sponsored study in Italy has begun exploring pediatric use, including the first child implanted with our device.


2025
The 24/7 SubQ system receives EU Approval for 3-Year Continuous Use of UNEEG SubQ Implant


2024
Winner of the Red dot award - Product design concept within the category Medical Devices and Technology


2024
EU MDR certificate achieved for the UNEEG SubQ implant.


2024
UNEEG EpiSight solution is granted FDA Breakthrough Device Designation within monitoring and diagnostic of epilepsy.


2024
Launch of UNEEG EpiSight – subcutaneous, ultra long-term, wireless EEG recording.


2022
UNEEG medical and King’s College London are awarded £1.8 million in the i4i Challenge Awards Call from the National Institute for Health and Care Research (NIHR) in the UK to conduct a multicentre, observational cohort study of the 24/7 EEG SubQ solution.


2022
Our UNEEG™ MyConnect data-transfer solution is launched. With MyConnect, for the first time EEG data are automatically sent from the patient to the hospital.


2021
First US patient initiated in pivotal multi-centre study for US approval of 24/7 EEG SubQ solution. The study is conducted at the University of Pennsylvania (US), Mayo Clinic (US) and Freiburg University Hospital (Germany)

2019
Pivotal study “ultra long-term subcutaneous home monitoring of epilepsy - 490 days of EEG from nine patients” is published in Epilepsia. The study is the first real-life monitoring of EEG in people with epilepsy; study participants record EEG data in their home environment with the 24/7 EEG SubQ. The study demonstrates that objective, real-life electrographic seizure counting can be obtained from subcutaneous EEG recordings. The study further concludes a substantial difference between the patient’s self-reported seizure counts and the objective seizure counts recorded with our solution.


2019
First UK patient implanted with the UNEEG SubQ as part of a USD 3 million programme funded by the Epilepsy Foundation of America as part of the project “My Seizure Gauge”.


2019
We receive ethics approval to initiate an ultra long-term sleep monitoring trial under the heading “Sleep in the ultra long-term perspective: Seasonally and behaviourally induced variation”.


2019
The 24/7 SubQ system is CE-marked, a major step forward in achieving our commercial ambitions.


2019
UNEEG launches a strategic partnership with the Austrian Institute of Technology, which obtains a CE-mark on a specialized 2-channel version of their encevis software that includes an algorithm for seizure detection.

2018
The study ”High similarity between EEG from subcutaneous and proximate scalp electrodes in patients with temporallobe epilepsy” is published, and shows that electrographic seizures can be monitored with the 24/7 SubQ.


2017
Our ultra long-term EEG technology receives the prestigious Shark Tank Prize from the US Epilepsy Foundation of America. The prize is awarded to the most innovative idea that can help those living with epilepsy.


2017
We change our name to UNEEG Medical.

2015
Pivotal paper ”EEG signal quality of a subcutaneous recording system compared to standard surface electrodes” shows that the signal quality of our implant at least equals that of scalp EEG.


2011
UNEEG conducts its first in man study - a major milestone, opening the path towards larger clinical trials.
2009
First epilepsy PhD research project is initiated under the title “Detection and Prediction of Epileptic Seizures”.


2009
Widex Holding becomes the majority shareholder of Hypo-Safe A/S. Widex Holding brings a vast experience in design, manufacturing and marketing of miniaturized medical devices to the company.


2005
The company Hypo-Safe A/S is founded by Professor dr. med. Henning Beck-Nielsen and co-founder, engineer Rasmus Stig Jensen. The original concept was designed to detect hypoglycemia in people with type 1 diabetes by monitoring brain activity. As the technology matured, the team discovered the implant could deliver high-quality signals over long periods - leading the founders to reassess where the technology could have the greatest clinical impact.

