News & Press Releases
UNEEG Medical marks first pediatric implantation in Europe-led study
1/8/2026
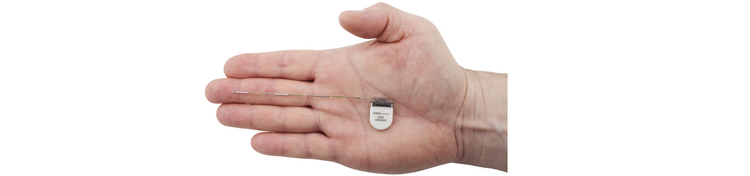
COPENHAGEN, January 8, 2026 – UNEEG Medical is proud to announce a groundbreaking milestone: for the first time, a child has been included in a clinical study using UNEEG’s ultra long-term brain monitoring solution.
UNEEG medical Achieves EU Approval for 3-Year Continuous Use of UNEEG SubQ Implant – Further Strengthening its EpiSight Solution for Epilepsy Monitoring
6/11/2025
COPENHAGEN, June 11, 2025 – UNEEG medical, a Danish company at the forefront of neuro- and epilepsy science, proudly announces the EU approval for 3-year continuous use of its subcutaneous implant, which is a significant milestone for the UNEEG EpiSight solution.
New CEO at UNEEG medical
3/7/2025
Martin Stenfeldt has taken over as new CEO of UNEEG medical. He succeeds Torben Sandgren, who has chosen to resign from his position after 7 years of dedicated service.
UNEEG receives FDA Breakthrough Device Designation
11/22/2024
The designation is a result of UNEEG’s strong focus on developing novel disease management support tools with transformational and first-to-market potential.
Red Dot Award to UNEEG medical
10/10/2024
UNEEG medical won the Red Dot Award for the design of UNEEG EpiSight.
UNEEG EpiSight launch
9/7/2024
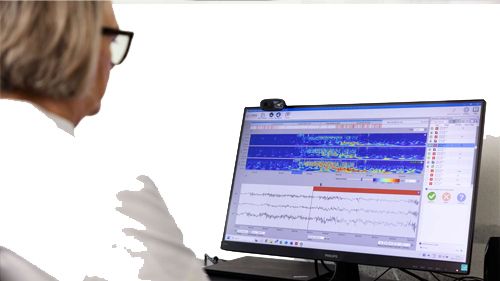
UNEEG medical announces world's first wireless, ultra long-term EEG recording of people with severe epilepsy – breakthrough features AI-supported analytics software for seizure and sleep correlation.
REAL-ASE Study
12/8/2023
We have reached an exciting milestone in the UK: Yesterday, the first patient in the REAL-ASE study had the 24/7 EEG™ SubQ implanted.
Policy Lab workshop
11/17/2023
Our colleagues were in London yesterday to participate at a Policy Lab workshop held at The Policy Institute at King’s College London.
Green Smiley to UNEEG medical
9/19/2023
UNEEG medical has received a Green Smiley from the Work Environment in Denmark.
New award for SubQ project in epilepsy
11/24/2022
UNEEG medical UK has received a £98,405 award for a SubQ EEG project in epilepsy aimed at people with intellectual disabilities or autism.
£1.8 million for real-world testing of 24/7 EEG SubQ
10/4/2022
UNEEG medical and King’s College London have been rewarded £1.8 million for real-world testing of our subcutaneous EEG solution.
Milestone in EEG sleep study
6/23/2022
Another great milestone in our landmark EEG sleep study has been reached.